
Upgrading Patient Controlled Analgesia Infusion Pumps
Looking to improve connectivity and safety, our medical device client wanted to upgrade their patient-controlled analgesia pump. Medical providers needed to program the devices with the correct medication and dosing for each patient.
In compliance with strict FDA regulations, we redesigned the pump to support wireless connectivity and improved the user experience. Our work reduced the chance of dosing errors.
What We Did
Tech Stack
Opportunity
A global pharmaceutical and infusion pump company needed to reduce use errors of an existing patient controlled analgesia infusion pump (a class II medical device). Hospitals needed a way to impose drug-specific dosing controls without having to configure each pump. Our client was also looking to expand the reach of their product to other countries.
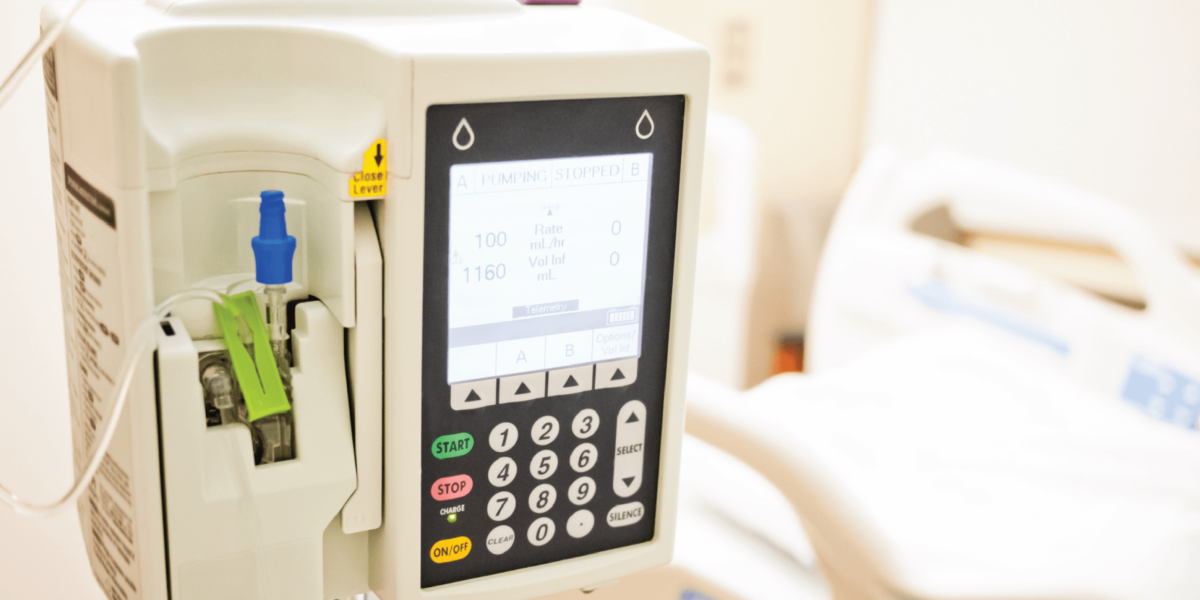
Solution
Our client engaged SEP to extend wireless connectivity to the pump in order to communicate with a single, hospital-wide, medication management system. We redesigned pump programming workflows to support dosing control limits received from the backend system. These limits alert the clinician to potential programming errors as well as limit how much medication is delivered. Wireless connectivity also allowed many other product improvements such as communication of real-time pump status information, automatic transfer of pump operation logs, remote pump configuration/setup, and field upgradability. Working closely with our client, SEP led activities to:
- Develop firmware to manage communication with centralized system through on-board custom network interfacing hardware
- Define and implement application level communication protocols utilizing XML-based messages
- Collaborate with client’s medical and marketing personnel to design and implement new pump capabilities, taking into consideration human factors issues
- Develop a bootloader for field upgradeability
- Add support for automatic programming of therapy settings
- Manage safety risks by contributing to hazard analysis and fault tree analysis
- Create all software design and verification documentation used in the 510(k) submission to the FDA
- Localize the product for the French-Canadian market and update the UI of the product to ensure easy addition of new languages in the future
Results
Our work together on this product resulted in a major advancement in infusion pump design: a reduction in dosing errors caused by nurse programming mistakes. There is no doubt that the new safety features of this product have helped avoid over-medication and saved patient lives. Our work allowed for international expansion of our client’s life saving product.
Recent Case Studies


Replacing an Excel Solution with Custom Software to Wow Clients and Financial Advisors

Implementing Enterprise DevOps Solutions
Let’s develop something special.
Reach out today to talk about how we can work together to shake up your industry.